
There is some dispute over the reaction of CO 2 (aq) with H 2O, with the possibility that the reactions areĬO 2 (aq) +2 H 2O H 3O + + HCO 3 − (slow) H 2O + CO 3 2- HCO 3 − + HO − as given by ab initio 6-311** calculationsĬarbon dioxide (CO 2) aqueous equilibria mouse over In addition to these equilibria, there should always be a balance of charges in solution (positive charges = negative charges), thus typically, Thus in alkaline solutions, the total concentration of carbonate carbon species ( + + ) increases considerably (mouse over the figure below right). These equilibria are shifted in alkaline solution (pH > ~5) where OH − ions remove the H 3O + ions H 2O + CO 2 H 2CO 3 catalyzed by HCO 3 − as given by ab initio 6-311** calculations.īicarbonate can similarly catalyze many hydrogen transfer reactions (e.g., keto-enol and imine-amine) PCO 2 is the partial pressure of CO 2 in the gas phase, atm. Calcium carbonate equilibria are given on the water descaling page.ġ is dissolved CO 2, mol ˣ L −1 and, if determined from solubility, also contains H 2CO 3, HCO 3 − and CO 3 2− The following equilibria occur (data at 25 ☌, with all constants changing with temperature) with some debate over the exact values of the constants. Varies with temperature ( see right) due to the higher CO 2 solubility at low temperatures and as described elsewhere. (≈ 0.26 %) to H 2CO 3 (O=C(OH) 2, see below right) in solution with the resulting

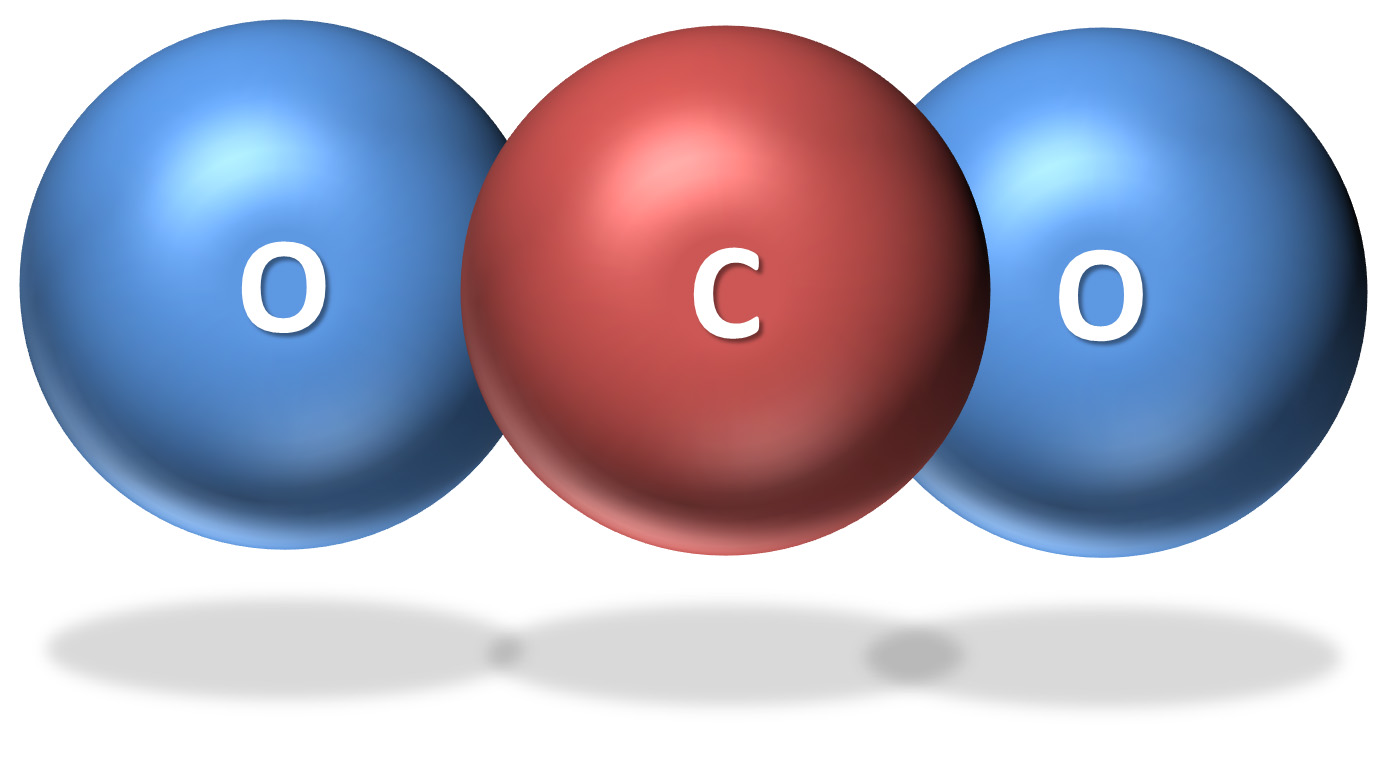
The common name for solid form of carbon dioxide is 'dry ice', first made commercially in 1925 by the DryIce Corporation of America. Adding dry ice to water causes a thick water fog to form from the liquid water. It has zero dipole moment with low thermal and electrical conductivity. Massive deposits of solid CO 2 have been discovered at Mars's south pole and appear to be flowing like glaciers on Earth. CO 2 is a linear molecule with the C=O bond length 0.114 nm and charges on the O and C atoms of -0.446 and + 0.892, respectively (using ab initio 6-311** calculation). CO 2 is very soluble in water with 3.346 grams of CO 2 dissolving in water at 0 ☌ (1.713 L CO2 gas ˣ L −1 liquid water at 0.10135 MPa partial pressure CO 2). Liquid CO 2 only forms above 0.51 MPa, liquid density 1101 kg ˣ m −3, -37 ☌ sublimation point, -78.5 ☌, 0.10135 MPa, solid density 1562 kg ˣ m −3 increasing with decreasing temperature triple point, 216.55 K, 518 kPa, gaseous density, 1.98 kg ˣ m −3 (at 0 ☌, 100 kPa) critical temperature 31.1 ☌, critical pressure 7.38 MPa, critical density 467.6 kg ˣ m −3, 13C content 1.1%. Carbon DioxideĬarbon dioxide (CO 2) is a colorless, odorless gas. This will not affect the tax due as Revenue will convert this to milligrams in the calculation at registration.It is difficult to remove traces of carbon dioxide from an aqueous solution and, once removed, aqueous solutions rapidly take up CO 2 from any exposure to the atmosphere.
Charge of carbon dioxide registration#
In the case of heavy duty vehicles this will be milligrams per kilowatt hour.ĭepending on documentation such as foreign registration certificates the figure may also be shown as grams per kilometre.
NOx is charged on the basis of milligrams per kilometre as recorded on the Certificate of Conformity. The NOx charge will be a component of vehicle registration tax as outlined below: Carbon Dioxide charge + NOx Charge = Total VRT payable

Nitrogen Oxide (NOx) emissions will be taxable beginning on 1 January 2020.The charge is applicable to all Category A vehicles excluding electrics but including hybrids.


 0 kommentar(er)
0 kommentar(er)
